56+ calculate zeff for a valence electron in an oxygen atom
Place the values for Z and S into the effective nuclear charge formula. Zeff Z S.

7 2 Shielding And Effective Nuclear Charge Chemistry Libretexts
There are more exact ways of determining Zeff which.

. S average amount of density between the nucleus and the. Web Effective nuclear charge Zeff is defined as A the number of protons minus the number of valence electrons B. A A valence electron in an oxygen atom b A valence electron in an iron atom c A 2p electron in an iron atom 7.
Web The Zeff for a valence electron in an oxygen atom is 455 The Zeff is the effective nuclear charge present in the number of protons existing within the nucleus. Calculate the effective nuclear charge on a valence electron in. In the above example for Na.
Web Calculate Zeff for the following. Find Z Effective Using Formula. Write the electron configuration of the atom in the following form.
It is given by the. Text H1s1 H 1s1 Hydrogen above has a single electron in the first shell. 11 88.
You have been asked to. There are more exact ways of determining Zeff which. 1s 2s 2p 3s 3p 3d 4s 4p 4d 4f 5s 5p.
S average amount of density between the nucleus and the electron. Web Z e f f can be calculated by subtracting the magnitude of shielding from the total nuclear charge and the effective nuclear charge of an atom is given by the. It is given by the.
Web Valence electrons are the electrons in the outermost shell or energy level of an atom. On the other hand helium has a. Web Slaters Rules.
Web Zeff the effective nuclear charge. Web The Zeff for a valence electron in an oxygen atom is 455 The Zeff is the effective nuclear charge present in the number of protons existing within the nucleus. Web In this class we will calculate Zeff Z S where S is the number of core electrons that are shielding the valence electrons.
For example oxygen has six valence electrons two in the 2s subshell and four in the 2p. Web Besides the formula for calculating the effective nuclear charge of a single electron is as follows. Z denotes the number of protons existing in the nucleus.
Web Lets take a look at the configuration for hydrogen. Web Step 5. Web In this class we will calculate Zeff Z S where S is the number of core electrons that are shielding the valence electrons.
Web Web The Zeff for a valence electron in an oxygen atom is 455 The Zeff is the effective nuclear charge present in the number of protons existing within the nucleus.
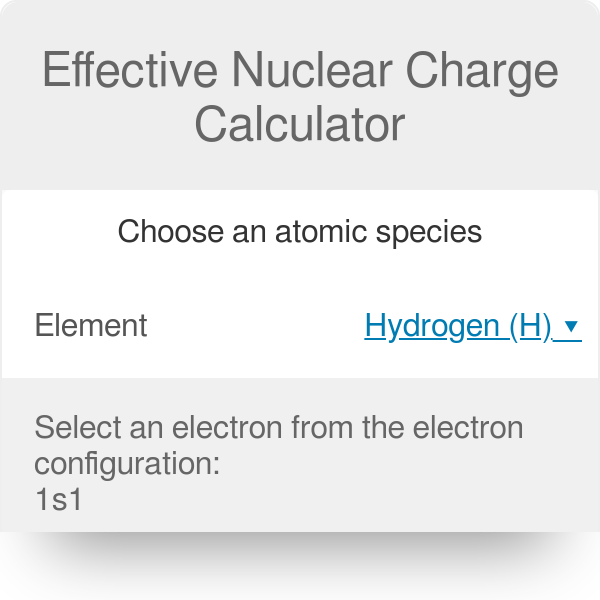
Effective Nuclear Charge Calculator Slater S Rule
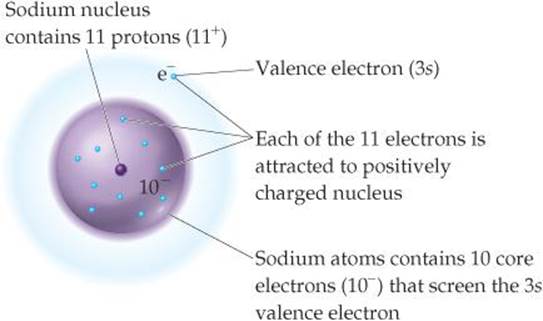
Effective Nuclear Charge Periodic Properties Of The Elements Chemistry The Central Science
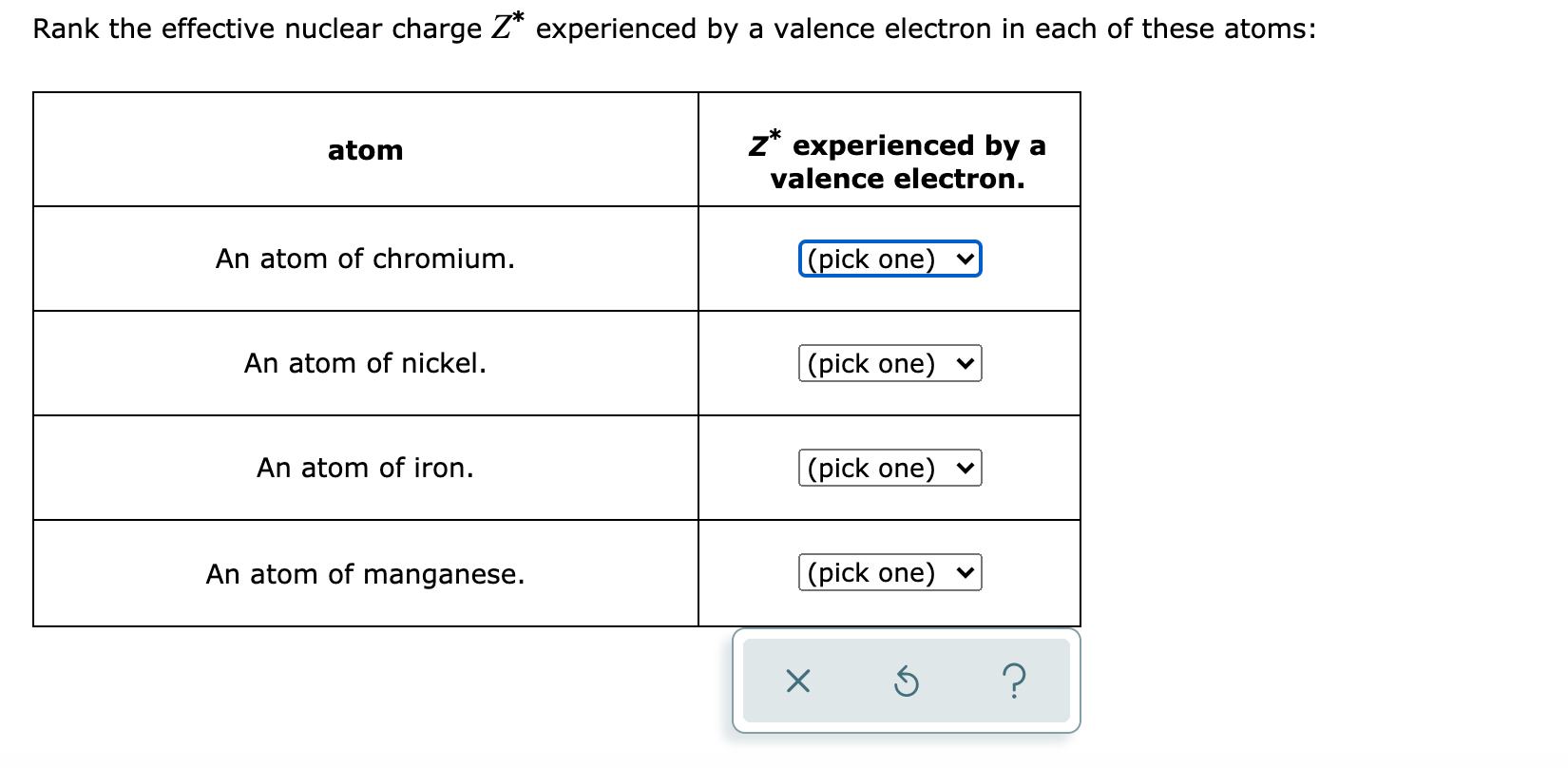
Answered Rank The Effective Nuclear Charge Z Bartleby

Calculate The Effective Nuclear Charge For The Outermost Electron Of Oxygen Atom

Solved Which Would Experience A Higher Effective Nuclear Chegg Com
How To Find The Effective Nuclear Charge Of Oxygen Quora
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
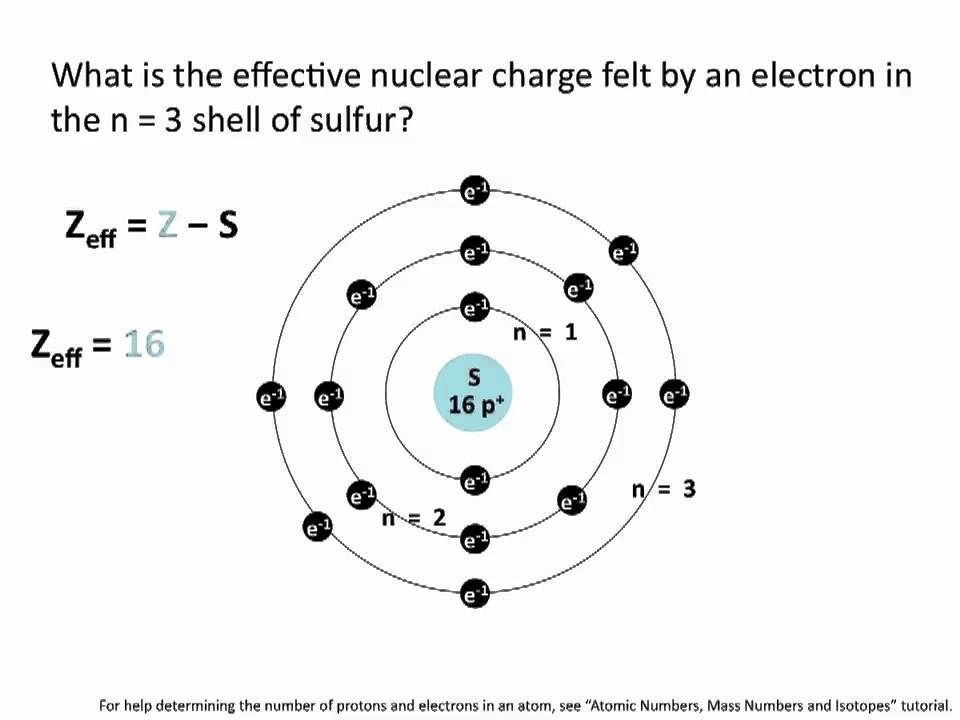
Effective Nuclear Charge Chemistry Tutorial Youtube
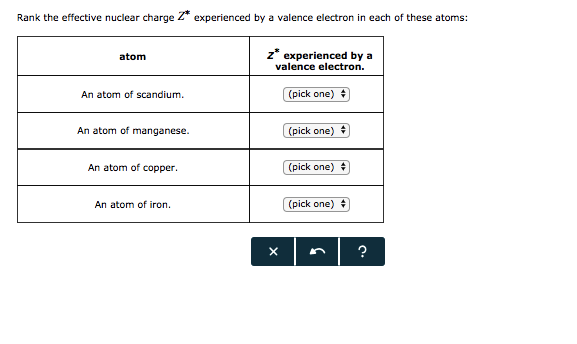
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Pdf Radios Ionicos De Shannon 5291 Fumou Duan Academia Edu
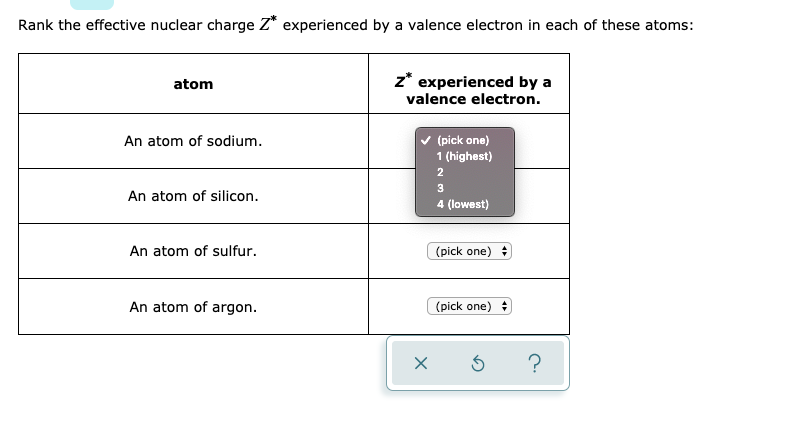
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
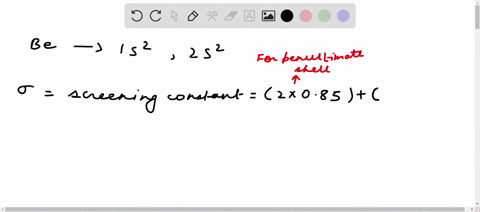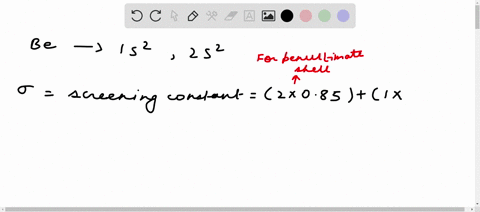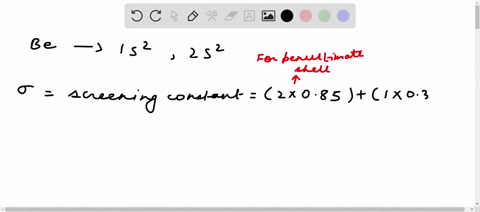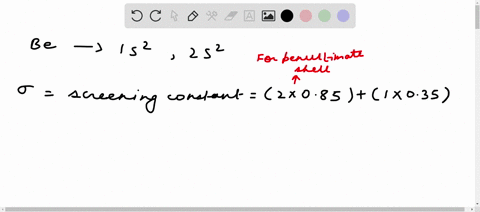
Solved Calculate The Effective Nuclear Charge On A Valence Electron In An Oxygen Atom

Effective Nuclear Charge
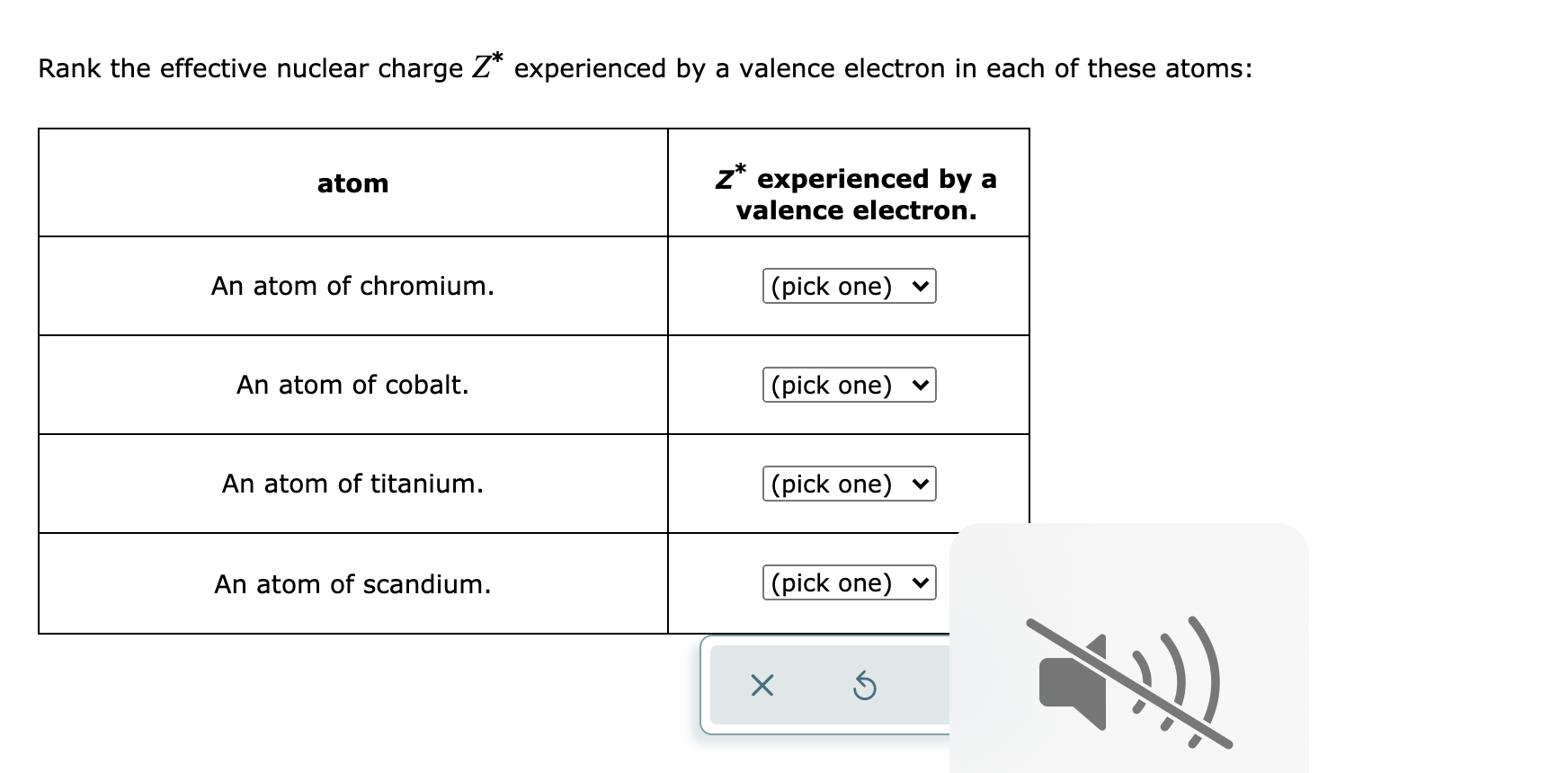
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com
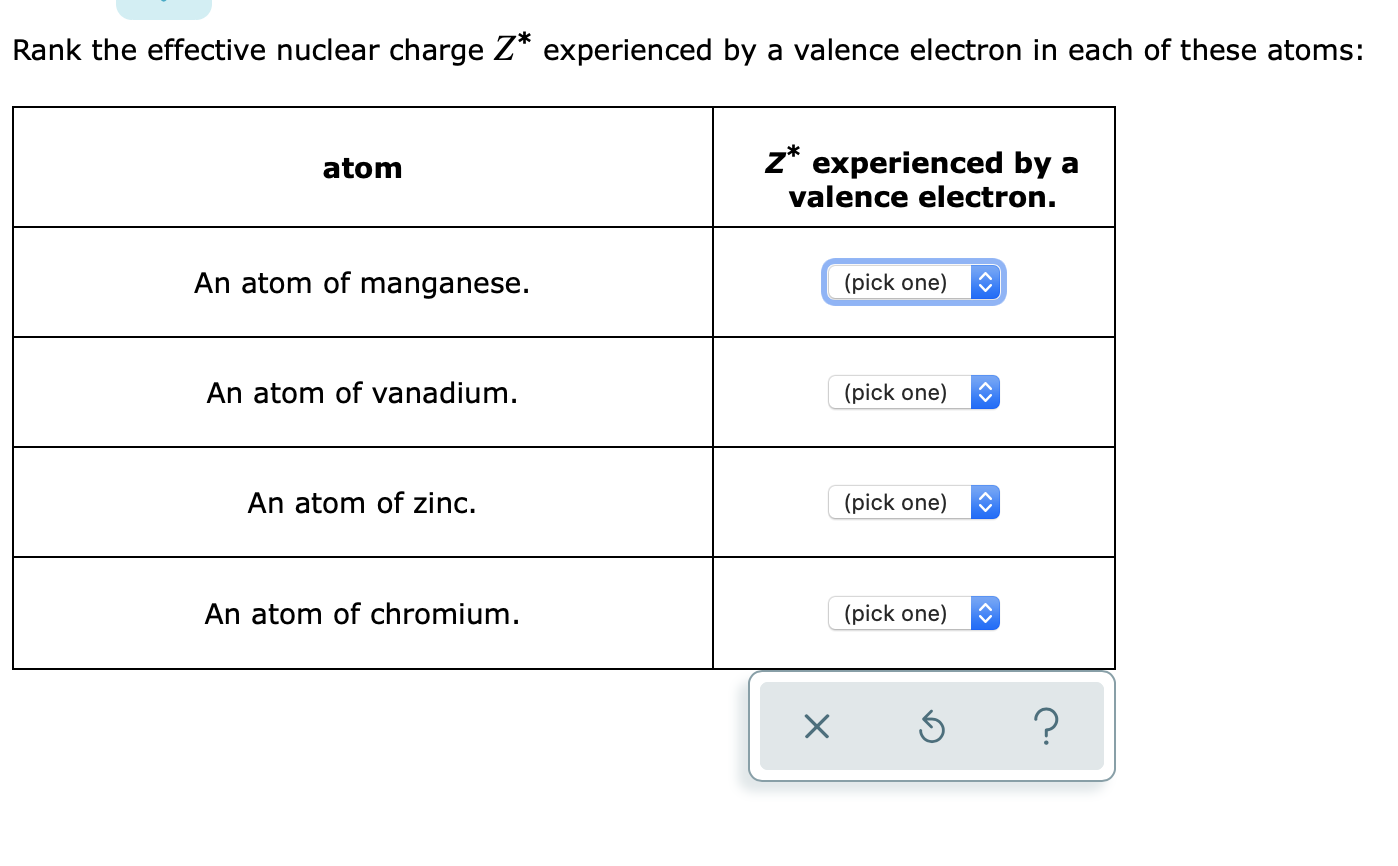
Solved Rank The Effective Nuclear Charge Z Experienced By A Chegg Com

Welcome To Chem Zipper Com Effective Nuclear Charge Z Or Zeff Slater S Rule Screening Effect Or Shielding Effect
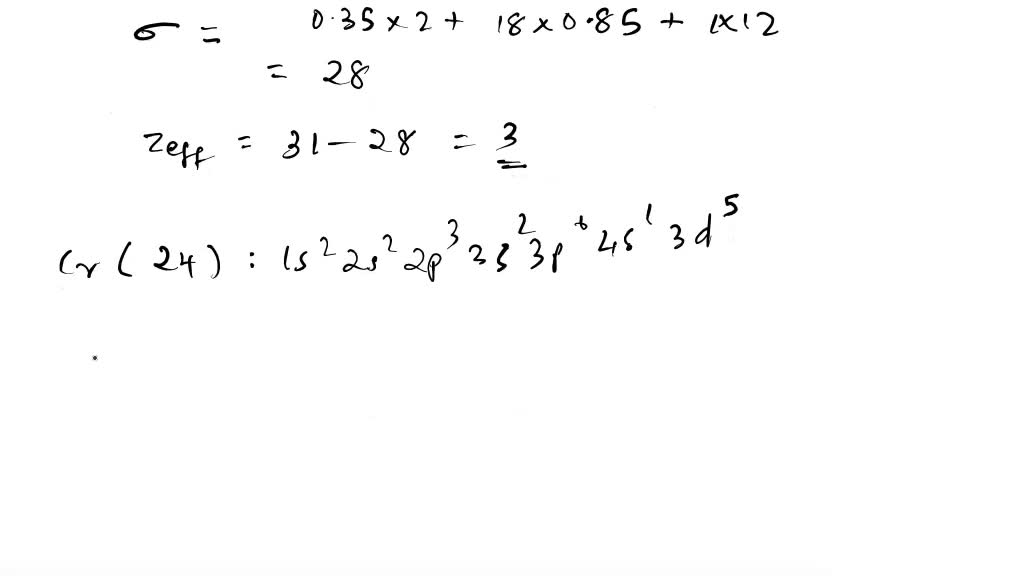
Solved Calculate Zeff For Oxygen Atom On Outermost Shell Electron Remember Contribution For Valance Electrons Are Ns And Np 0 35 N 1 Contribution Is 0 85 The Second Ionization Energy Of Some Period Elements Are